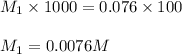Chemistry, 24.07.2019 22:20 canyonrico05
5.00 ml is withdrawn from a 1.00l solution of nacl and diluted to 100.00 ml. if the concentration of the nacl in the 100.00 ml solution is 0.076, what is the concentration of nacl in the original 1.00 l solution? answers

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

You know the right answer?
5.00 ml is withdrawn from a 1.00l solution of nacl and diluted to 100.00 ml. if the concentration of...
Questions


Biology, 19.01.2021 07:10


Biology, 19.01.2021 07:10


Social Studies, 19.01.2021 07:10

History, 19.01.2021 07:10



Chemistry, 19.01.2021 07:10


English, 19.01.2021 07:10


Computers and Technology, 19.01.2021 07:10

Health, 19.01.2021 07:10




English, 19.01.2021 07:10

Mathematics, 19.01.2021 07:10

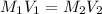
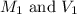 are the molarity and volume of the concentrated solution
are the molarity and volume of the concentrated solution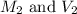 are the molarity and volume of diluted solution
are the molarity and volume of diluted solution