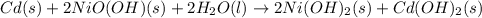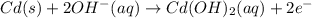Chemistry, 24.07.2019 21:10 Looneytunness1806
Anicd battery uses nickel and cadmium to produce a potential difference. using these equations, answer the following questions. i. 2nio(oh) (s) + 2h 2o (l) + 2 e - → 2ni(oh) 2 (s) + 2 oh¯ (aq) ii. cd (s) + 2oh¯ (aq) → cd(oh) 2 (s) + 2e¯ iii. cd (s) + 2nio(oh) (s) + 2 h 2o (l) → 2 ni(oh) 2 (s) + cd(oh) 2 (s) which equation represents the whole chemical reaction within the galvanic cell? i ii iii i and ii

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
Anicd battery uses nickel and cadmium to produce a potential difference. using these equations, answ...
Questions



English, 25.04.2020 03:07










History, 25.04.2020 03:07


Mathematics, 25.04.2020 03:07



Mathematics, 25.04.2020 03:07