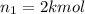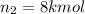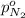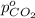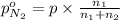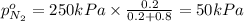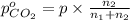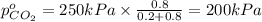Chemistry, 24.07.2019 19:10 holasoykawaii10
Arigid tank is divided into two equal volumes. one side contains 2 kmol of nitrogen n2 at 500 kpa while the other side contains 8 kmol of co2 at 200 kpa. the two sides are now connected and the gases are mixed and forming a homogeneous mixture at 250 kpa. find the partial pressure of the co2 in the final mixture.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1

Chemistry, 23.06.2019 10:30
Ethyl alcohol, also known as ethanol, has a density of 0.79 g/ml. what is the volume, in quarts, of 1.95 kg of this alcohol?
Answers: 2

Chemistry, 23.06.2019 13:00
Sort these isotopes by whether they are most likely to undergo fusion or fission. hydrogen-3, uranium-233, plutonium-239, hydrogen-1, helium-3, plutonium-241
Answers: 2
You know the right answer?
Arigid tank is divided into two equal volumes. one side contains 2 kmol of nitrogen n2 at 500 kpa wh...
Questions

Mathematics, 29.10.2020 18:20

Engineering, 29.10.2020 18:20

Chemistry, 29.10.2020 18:20

Computers and Technology, 29.10.2020 18:20

English, 29.10.2020 18:20



Mathematics, 29.10.2020 18:20


Computers and Technology, 29.10.2020 18:20

Chemistry, 29.10.2020 18:20

Mathematics, 29.10.2020 18:20

English, 29.10.2020 18:20



Physics, 29.10.2020 18:20



Mathematics, 29.10.2020 18:20

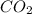 in the final mixture is 200 kPa.
in the final mixture is 200 kPa.