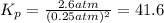Chemistry, 24.07.2019 17:10 devilrao6742
The reaction x 2 (g) m 2 x(g) occurs in a closed reaction vessel at constant volume and temperature. initially, the vessel contains only x 2 at a pressure of 1.55 atm. after the reaction reaches equilibrium, the total pressure is 2.85 atm. what is the value of the equilibrium constant, kp , for the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3

You know the right answer?
The reaction x 2 (g) m 2 x(g) occurs in a closed reaction vessel at constant volume and temperature....
Questions

Mathematics, 18.12.2019 08:31

Physics, 18.12.2019 08:31

Social Studies, 18.12.2019 08:31

Mathematics, 18.12.2019 08:31

Health, 18.12.2019 08:31

History, 18.12.2019 08:31




Physics, 18.12.2019 08:31

Biology, 18.12.2019 08:31

Mathematics, 18.12.2019 08:31

Mathematics, 18.12.2019 08:31

English, 18.12.2019 08:31

Health, 18.12.2019 08:31

Mathematics, 18.12.2019 08:31




English, 18.12.2019 08:31

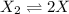
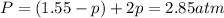
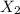 at equilibrium:
at equilibrium:![[p_{X_2}^o]=2p=2\time 1.3 atm=2.6 atm](/tpl/images/0128/0119/e4252.png)
![[p_{X}^{o}]=1.55 atm -1.3 atm = 0.25 atm](/tpl/images/0128/0119/f5d78.png)
![K_p=\frac{[p_{X_2}^o]}{[p_{X}^{o}]^2}](/tpl/images/0128/0119/aa6e2.png)