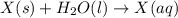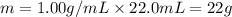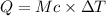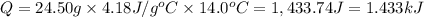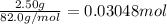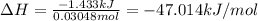Chemistry, 24.07.2019 16:10 christhegreat1
Acalorimeter contains 22.0 ml of water at 14.0 ∘c . when 2.50 g of x (a substance with a molar mass of 82.0 g/mol ) is added, it dissolves via the reaction x(s)+h2o(l)→x(aq) and the temperature of the solution increases to 28.0 ∘c . calculate the enthalpy change, δh, for this reaction per mole of x. assume that the specific heat of the resulting solution is equal to that of water [4.18 j/(g⋅∘c)], that density of water is 1.00 g/ml, and that no heat is lost to the calorimeter itself, nor to the surroundings.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
Acalorimeter contains 22.0 ml of water at 14.0 ∘c . when 2.50 g of x (a substance with a molar mass...
Questions

Mathematics, 15.01.2020 13:31

Mathematics, 15.01.2020 13:31



Mathematics, 15.01.2020 13:31



Mathematics, 15.01.2020 13:31

Social Studies, 15.01.2020 13:31





Mathematics, 15.01.2020 13:31



Mathematics, 15.01.2020 13:31

Mathematics, 15.01.2020 13:31

Mathematics, 15.01.2020 13:31