

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 23.06.2019 15:00
How many more valence electrons does sodium need to have a full outer valence shell
Answers: 3

Chemistry, 23.06.2019 17:30
Two examples of energy transformations are shown. the energy transformations are similar because they both involve transformations that begin with chemical energy. begin with electrical energy. result in radiant energy. result in mechanical energy.
Answers: 2
You know the right answer?
Where the oxygen comes from the air (21% o2 and 79% n2). if oxygen is fed from air in excess of the...
Questions

Mathematics, 20.12.2019 19:31




Computers and Technology, 20.12.2019 19:31



Social Studies, 20.12.2019 19:31

Business, 20.12.2019 19:31

History, 20.12.2019 19:31

Mathematics, 20.12.2019 19:31

Computers and Technology, 20.12.2019 19:31

Mathematics, 20.12.2019 19:31




Mathematics, 20.12.2019 19:31

Spanish, 20.12.2019 19:31

Advanced Placement (AP), 20.12.2019 19:31

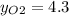 %
%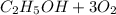 →
→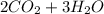
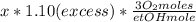
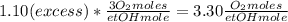
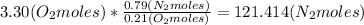
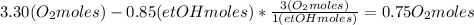
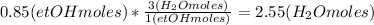
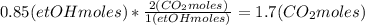
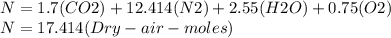
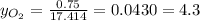 %
%

