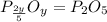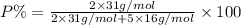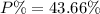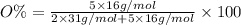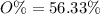Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1

Chemistry, 23.06.2019 05:00
110 g of water (specific heat = 4.184 j/g c) and 100 g of a metal sample (specific heat = 0.397 j/g c) are heated from 25 degrees c to 75 degrees c. which substance required more thermal energy?
Answers: 1

Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1
You know the right answer?
When a 35.07 g sample of phosphorus reacts with oxygen a 71.00 g sample of phosphorus oxide is forme...
Questions


Social Studies, 07.11.2019 06:31

History, 07.11.2019 06:31


Physics, 07.11.2019 06:31





Mathematics, 07.11.2019 06:31

History, 07.11.2019 06:31


History, 07.11.2019 06:31

Health, 07.11.2019 06:31

Mathematics, 07.11.2019 06:31


English, 07.11.2019 06:31


History, 07.11.2019 06:31

Mathematics, 07.11.2019 06:31

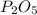 is the empirical formula for this compound.
is the empirical formula for this compound.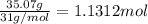
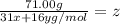
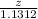 moles of phosphorus oxide...(1)
moles of phosphorus oxide...(1)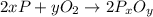
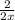 moles of phosphorus oxide...(2)
moles of phosphorus oxide...(2)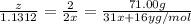
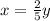
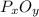 :
: