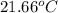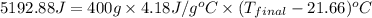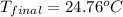Chemistry, 22.07.2019 23:20 autumnguidry7628
Aquantity of 2.00 × 102 ml of 0.461 m hcl is mixed with 2.00 × 102 ml of 0.231 m ba(oh)2 in a constant-pressure calorimeter of negligible heat capacity. the initial temperature of the hcl and ba(oh)2 solutions is the same at 21.66°c. for the process below, the heat of neutralization is −56.2 kj/mol. what is the final temperature of the mixed solutions? h+(aq) + oh−(aq) → h2o(l)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 23.06.2019 04:00
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1

Chemistry, 23.06.2019 11:30
How do you calculate the mass of a product when the amounts of more than one reactant are given?
Answers: 3
You know the right answer?
Aquantity of 2.00 × 102 ml of 0.461 m hcl is mixed with 2.00 × 102 ml of 0.231 m ba(oh)2 in a consta...
Questions

Mathematics, 12.07.2019 10:00

Mathematics, 12.07.2019 10:00


Health, 12.07.2019 10:00

English, 12.07.2019 10:00

Mathematics, 12.07.2019 10:00

Social Studies, 12.07.2019 10:00


Social Studies, 12.07.2019 10:00


Social Studies, 12.07.2019 10:00

Mathematics, 12.07.2019 10:00

Mathematics, 12.07.2019 10:00


Biology, 12.07.2019 10:00




Mathematics, 12.07.2019 10:00

Mathematics, 12.07.2019 10:00

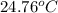
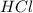 and
and 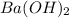 .
.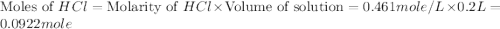
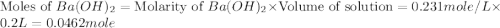
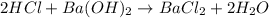
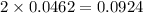 mole of HCl
mole of HCl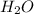
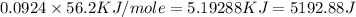
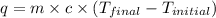
 = specific heat capacity =
= specific heat capacity = 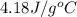
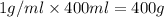
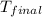 = final temperature = ?
= final temperature = ?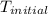 = initial temperature =
= initial temperature =