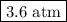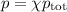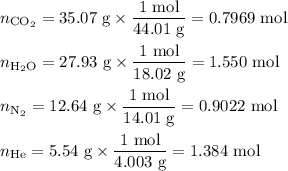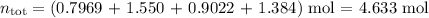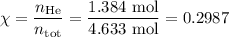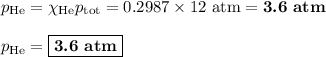Chemistry, 22.07.2019 05:10 tiwaribianca475
Amixture contains 35.07 grams of carbon dioxide, 27.93 grams of water vapor, 12.64 grams of nitrogen, and 5.54 grams of helium. the total pressure of the system is 12 atm, what is the partial pressure of the helium?
a. 0.88 atm
b. 0.82 atm
c. 0.073 atm
d. 0.068 atm

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
Amixture contains 35.07 grams of carbon dioxide, 27.93 grams of water vapor, 12.64 grams of nitrogen...
Questions


Physics, 14.10.2019 18:30




Mathematics, 14.10.2019 18:30

Social Studies, 14.10.2019 18:30


Chemistry, 14.10.2019 18:30


Arts, 14.10.2019 18:30


Geography, 14.10.2019 18:30

English, 14.10.2019 18:30

Biology, 14.10.2019 18:30

Chemistry, 14.10.2019 18:30


English, 14.10.2019 18:30


Mathematics, 14.10.2019 18:30