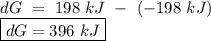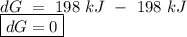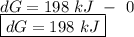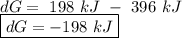1point
for a reaction, a h= 198 kj. for which value of ta sis the reaction
spontaneous?...

Chemistry, 21.07.2019 01:10 savyblue1724707
1point
for a reaction, a h= 198 kj. for which value of ta sis the reaction
spontaneous?
o a. -198 kj
o b. 198 kj
o c. oku
o d. 396 kj
submit

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1


Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
Questions

History, 18.05.2020 22:58




Mathematics, 18.05.2020 22:58

Chemistry, 18.05.2020 22:58

History, 18.05.2020 22:58


Mathematics, 18.05.2020 22:58





Chemistry, 18.05.2020 22:58

Mathematics, 18.05.2020 22:58


Mathematics, 18.05.2020 22:58

Mathematics, 18.05.2020 22:58

Chemistry, 18.05.2020 22:58

Mathematics, 18.05.2020 22:58

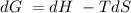 A positive ΔG represents a non-spontaneous reactionA negative ΔG value indicates a spontaneous reaction.
A positive ΔG represents a non-spontaneous reactionA negative ΔG value indicates a spontaneous reaction.