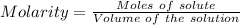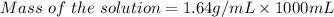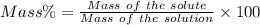Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
The density of an aqueous solution of nitric acid is 1.64 g/ml and the concentration is 1.85 m. what...
Questions


History, 28.06.2019 20:30

History, 28.06.2019 20:30

Social Studies, 28.06.2019 20:30

History, 28.06.2019 20:30

Business, 28.06.2019 20:30




Chemistry, 28.06.2019 20:30


Mathematics, 28.06.2019 20:30


Biology, 28.06.2019 20:30



History, 28.06.2019 20:30

Mathematics, 28.06.2019 20:30

Chemistry, 28.06.2019 20:30

Business, 28.06.2019 20:30