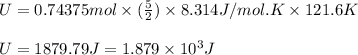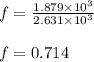Chemistry, 20.07.2019 02:30 aubriebv2020
Suppose 23.8 g of oxygen (o2) is heated at constant atmospheric pressure from 27.4°c to 149°c. (a) how many moles of oxygen are present? (take the molar mass of oxygen to be 32.0 g/mol) (b) how much energy is transferred to the oxygen as heat? (the molecules rotate but do not oscillate.) (c) what fraction of the heat is used to raise the internal energy of the oxygen?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3


Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
You know the right answer?
Suppose 23.8 g of oxygen (o2) is heated at constant atmospheric pressure from 27.4°c to 149°c. (a) h...
Questions

Chemistry, 10.03.2021 22:20


Social Studies, 10.03.2021 22:20

English, 10.03.2021 22:20

Mathematics, 10.03.2021 22:20


Mathematics, 10.03.2021 22:20


Mathematics, 10.03.2021 22:20

Mathematics, 10.03.2021 22:20

World Languages, 10.03.2021 22:20

Biology, 10.03.2021 22:20

Mathematics, 10.03.2021 22:20




Physics, 10.03.2021 22:20


Mathematics, 10.03.2021 22:20

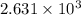
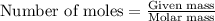
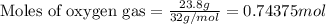
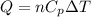
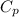 = specific heat capacity at constant pressure =
= specific heat capacity at constant pressure = 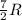 (For diatomic gas)
(For diatomic gas) = change in temperature =
= change in temperature = 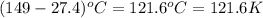
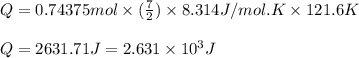
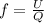
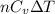
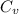 = specific heat capacity at constant pressure =
= specific heat capacity at constant pressure = 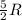 (For diatomic gas)
(For diatomic gas)