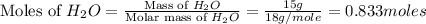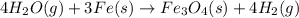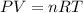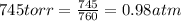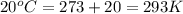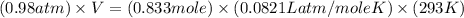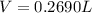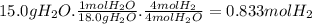Cavendish prepared hydrogen in 1766 by the novel method of passing steam through a red-hot gun barrel: 4h2 o(g) + 3fe(s) ⟶ fe3 o4 (s) + 4h2 (g) (a) outline the steps necessary to answer the following question: what volume of h2 at a pressure of 745 torr and a temperature of 20 °c can be prepared from the reaction of 15.o g of h2o? (b) answer the question.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2


Chemistry, 23.06.2019 04:20
Which activity describes an application of topographic maps? check all that apply. recreation, such as camping and hiking engineering, such as the construction of roads and buildings science, such as mapping stars in the sky business, such as analyzing population centers science, such as analyzing surface features
Answers: 1

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1
You know the right answer?
Cavendish prepared hydrogen in 1766 by the novel method of passing steam through a red-hot gun barre...
Questions


Mathematics, 24.10.2020 19:30


Mathematics, 24.10.2020 19:30

Mathematics, 24.10.2020 19:30

Mathematics, 24.10.2020 19:30


Mathematics, 24.10.2020 19:30


Geography, 24.10.2020 19:30




Health, 24.10.2020 19:30

History, 24.10.2020 19:30

Mathematics, 24.10.2020 19:30



Health, 24.10.2020 19:30

History, 24.10.2020 19:30

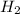 will be, 0.2690 L
will be, 0.2690 L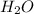 .
.