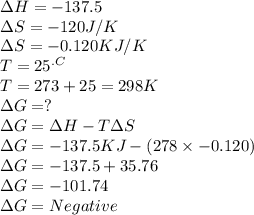Chemistry, 19.07.2019 22:20 haleybain6353
C2h4(g) + h2(g) → c2h6(g) δh = –137.5 kj; δs = –120.5 j/k calculate δg at 25 °c and determine whether the reaction is spontaneous. does δg become more negative or more positive as the temperature increases?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which of the following mining methods disrupts the sea floor?
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
You know the right answer?
C2h4(g) + h2(g) → c2h6(g) δh = –137.5 kj; δs = –120.5 j/k calculate δg at 25 °c and determine wheth...
Questions

History, 25.09.2020 14:01









English, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01


Physics, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01


Mathematics, 25.09.2020 14:01

Arts, 25.09.2020 14:01