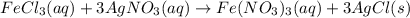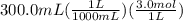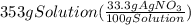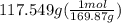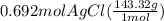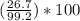Chemistry, 19.07.2019 20:10 laurielaparr2930
300. ml of a 3.0 m aqueous solution of iron (iii) chloride is mixed with 353 grams of a 33.3 mass % solution of silver (u) nitrate and water. 267 grams of a solid precipitate forms. what is the percent yield of the reaction assuming that the solubility of the solid precipitate in water is negligible.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 23.06.2019 12:40
During an experiment, ice and water were placed in a perfectly insulated thermos flask at 0 °c. describe this system when it phase reaches equilibrium.
Answers: 1
You know the right answer?
300. ml of a 3.0 m aqueous solution of iron (iii) chloride is mixed with 353 grams of a 33.3 mass %...
Questions

Mathematics, 04.06.2021 06:10

Mathematics, 04.06.2021 06:10


Mathematics, 04.06.2021 06:10

Mathematics, 04.06.2021 06:10


Mathematics, 04.06.2021 06:10


Mathematics, 04.06.2021 06:10




Mathematics, 04.06.2021 06:10

Physics, 04.06.2021 06:10


Mathematics, 04.06.2021 06:10

Chemistry, 04.06.2021 06:10



Mathematics, 04.06.2021 06:10