Chemistry, 19.07.2019 05:20 kailahgranger
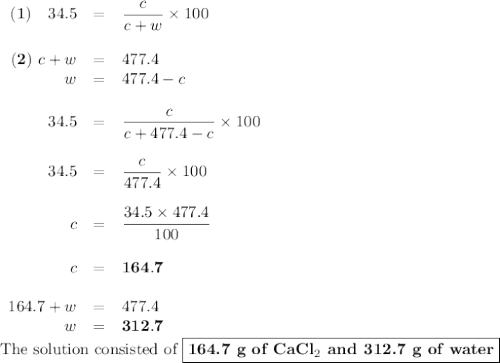
Asolution of cacl2 in water forms a mixture that is 34.5% calcium chloride by mass. if the total mass of the mixture is 477.4 g, what masses of cacl2 and water were used?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1


Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
You know the right answer?
Asolution of cacl2 in water forms a mixture that is 34.5% calcium chloride by mass. if the total mas...
Questions












English, 27.10.2020 22:20

Mathematics, 27.10.2020 22:20

Mathematics, 27.10.2020 22:20

Chemistry, 27.10.2020 22:20

Mathematics, 27.10.2020 22:20

History, 27.10.2020 22:20

World Languages, 27.10.2020 22:20

History, 27.10.2020 22:20