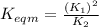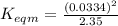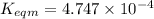Chemistry, 19.07.2019 04:30 yarielisr18
Use the reactions below and their equilibrium constants to predict the equilibrium constant for the reaction 2a(s)⇌3d(g). a(s) ⇌ 12 b(g)+c(g), k1=0.0334 3d(g) ⇌ b(g)+2c(g), k2=2.35

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

You know the right answer?
Use the reactions below and their equilibrium constants to predict the equilibrium constant for the...
Questions

Mathematics, 25.10.2019 09:43

Chemistry, 25.10.2019 09:43

Biology, 25.10.2019 09:43


Social Studies, 25.10.2019 09:43

Health, 25.10.2019 09:43







History, 25.10.2019 09:43

Biology, 25.10.2019 09:43

Mathematics, 25.10.2019 09:43

History, 25.10.2019 09:43

Health, 25.10.2019 09:43



Mathematics, 25.10.2019 09:43


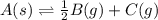
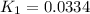
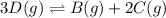

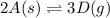
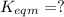
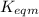 for the final reaction.
for the final reaction.