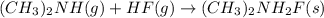Chemistry, 19.07.2019 03:20 Brookwiggington8814
Using the lewis concept of acids and bases, identify the lewis acid and base in each of the following reactions: ni(no3)3(s) 6h2o(l)→ni(h2o)63 (aq) 3no3−(aq) (ch3)2nh(g) hf(g)→(ch3)2nh2f(s)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2
You know the right answer?
Using the lewis concept of acids and bases, identify the lewis acid and base in each of the followin...
Questions


Social Studies, 23.10.2019 07:00




Mathematics, 23.10.2019 07:00

Mathematics, 23.10.2019 07:00


Biology, 23.10.2019 07:00

Social Studies, 23.10.2019 07:00

Mathematics, 23.10.2019 07:00

Mathematics, 23.10.2019 07:00



Geography, 23.10.2019 07:00

Mathematics, 23.10.2019 07:00



Mathematics, 23.10.2019 07:00

Biology, 23.10.2019 07:00

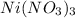 and Lewis-base =
and Lewis-base = 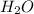
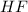 and Lewis-base =
and Lewis-base = 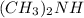 [/tex]
[/tex]![Ni(NO_3)_3(s)+6H_2O(l)\rightarrow [Ni(H_2O)_6]^{3+}(aq)+3NO_3^-(aq)](/tpl/images/0106/3688/a6dfb.png)
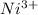 which is a Lewis-acid.
which is a Lewis-acid.