
Chemistry, 18.07.2019 23:30 kalialee2424
How many grams of nahco3 (fm 84.01 g/mol) should be mixed with na2co3 to produce a 1.00 l buffer solution with ph 9.50. the final concentration of na2co3 in this solution is 0.10 m. pka1 = 6.37 and pka2 = 10.33 for h2co3.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
How many grams of nahco3 (fm 84.01 g/mol) should be mixed with na2co3 to produce a 1.00 l buffer sol...
Questions



Mathematics, 18.03.2021 03:10











Mathematics, 18.03.2021 03:10

Biology, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10



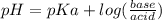
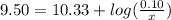
 is the concentration of sodium bicarbonate)
is the concentration of sodium bicarbonate)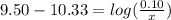
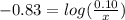
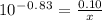
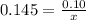
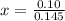
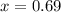
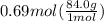
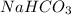 will be required.
will be required. 

