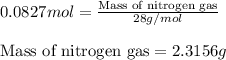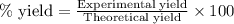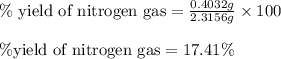Chemistry, 18.07.2019 22:10 jgstyle2388
Hydrazine, n2h4 , reacts with oxygen to form nitrogen gas and water. n2h4(aq)+o2(g)⟶n2(g)+2h2o(l) if 2.65 g of n2h4 reacts with excess oxygen and produces 0.350 l of n2 , at 295 k and 1.00 atm, what is the percent yield of the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

You know the right answer?
Hydrazine, n2h4 , reacts with oxygen to form nitrogen gas and water. n2h4(aq)+o2(g)⟶n2(g)+2h2o(l) if...
Questions



Chemistry, 04.03.2021 02:10

History, 04.03.2021 02:10

Social Studies, 04.03.2021 02:10

Mathematics, 04.03.2021 02:10

Mathematics, 04.03.2021 02:10


Mathematics, 04.03.2021 02:10


Mathematics, 04.03.2021 02:10

History, 04.03.2021 02:10

Mathematics, 04.03.2021 02:10


English, 04.03.2021 02:10



Mathematics, 04.03.2021 02:10

Chemistry, 04.03.2021 02:10

Physics, 04.03.2021 02:10

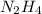
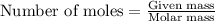
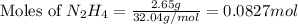
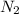
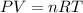
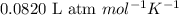
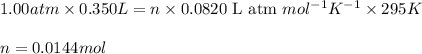
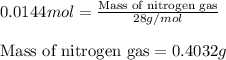
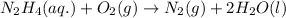
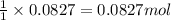 of nitrogen gas.
of nitrogen gas.