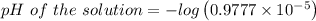Chemistry, 18.07.2019 20:30 loveniasummer71
Ammonium chloride, nh4cl, is a salt formed from the neutralization of the weak base ammonia, nh3, with the strong acid hydrochloric acid. given that the value of kb for ammonia is 1.8×10−5, what is the ph of a 0.176 m solution of ammonium chloride at 25∘c?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
Ammonium chloride, nh4cl, is a salt formed from the neutralization of the weak base ammonia, nh3, wi...
Questions




Mathematics, 03.12.2020 23:40

Law, 03.12.2020 23:40


Health, 03.12.2020 23:40

English, 03.12.2020 23:40







History, 03.12.2020 23:40


Physics, 03.12.2020 23:40

Mathematics, 03.12.2020 23:40


![\left[H^+ \right]=\sqrt{K_a\times C}](/tpl/images/0105/2520/72ad6.png)
![\left[H^+ \right]=\sqrt{5.5556\times 10^{-10}\times 0.176}](/tpl/images/0105/2520/b4744.png)
![\left[H^+ \right]=0.9777\times 10^{-5}](/tpl/images/0105/2520/5c7e7.png)
![pH\ of\ the\ solution=-log\left[H^+ \right]](/tpl/images/0105/2520/ec689.png)