
Chemistry, 12.11.2019 12:31 thederpbro
Acompound has the formula ch3co2h. r how should the relative molecular mass, mr, of this compound be calculated? a 12 1 16b 3(12 1) 2(12 16) 1c (4 x 12) (2 x 1) 16d (2 x 12) (4 x 1) (2 x 16)
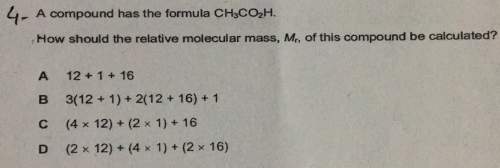

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1
You know the right answer?
Acompound has the formula ch3co2h. r how should the relative molecular mass, mr, of this compound be...
Questions

English, 28.07.2019 02:40



Biology, 28.07.2019 02:40



Mathematics, 28.07.2019 02:40

History, 28.07.2019 02:40

Mathematics, 28.07.2019 02:40

Mathematics, 28.07.2019 02:40


Advanced Placement (AP), 28.07.2019 02:40





Health, 28.07.2019 02:40

Biology, 28.07.2019 02:40

History, 28.07.2019 02:40

Chemistry, 28.07.2019 02:40




