
Chemistry, 28.08.2019 17:30 themaster66644
If two gases are present in a container, the total pressure in the container is equal to
a. the sum of the pressures that are exerted by each of the two gases.
b. twice the sum of the pressures that are exerted by the individual gases.
c. the sum of the pressures that each gas would exert if they occupied twice the volume.
d. the sum of the pressures that each gas would exert if they occupied half the volume.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
If two gases are present in a container, the total pressure in the container is equal to
a. th...
a. th...
Questions


















Mathematics, 19.06.2020 03:57


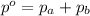
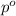 = Total pressure exerted by the gases in the container.
= Total pressure exerted by the gases in the container.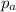 = Partial pressure of gas 'a'
= Partial pressure of gas 'a'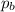 = partial pressure of gas 'b'
= partial pressure of gas 'b'


