
Chemistry, 16.07.2019 21:20 monkeys450
Asimple way to estimate whether a molecule is a strong electrolyte, a weak electrolyte, or a nonelectrolyte is to examine an equation that accurately describes its behavior in water. when examining the chemical equations, start by looking at the direction and type of reaction arrow. a chemical equation reported in the literature may include a forward reaction arrow (→), equilibrium reaction arrow (⇌), or reverse reaction arrow (←). next, determine whether ions are likely to be present and, if present, in what quantities they should be present.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3


Chemistry, 23.06.2019 13:00
What type of reaction is this equation c2h5s + o2 > co2 + h2o + so2
Answers: 2
You know the right answer?
Asimple way to estimate whether a molecule is a strong electrolyte, a weak electrolyte, or a nonelec...
Questions

English, 14.10.2020 23:01

Physics, 14.10.2020 23:01

Chemistry, 14.10.2020 23:01

Mathematics, 14.10.2020 23:01



English, 14.10.2020 23:01

English, 14.10.2020 23:01


History, 14.10.2020 23:01

Mathematics, 14.10.2020 23:01




English, 14.10.2020 23:01

Mathematics, 14.10.2020 23:01



Social Studies, 14.10.2020 23:01

Law, 14.10.2020 23:01

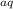 → Na⁺
→ Na⁺

