
Chemistry, 16.07.2019 12:10 kinziemadison12
Asample of cesium carbonate, weighing 3.80 g, requires 1.90 g of hydrogen bromide gas to completely decompose to water, cesium bromide, and carbon dioxide gas. the total mass of water and cesium bromide formed is 5.20 g and no hydrogen bromide or cesium carbonate remains. according to the law of conservation of mass, what mass of carbon dioxide must have been formed?
a. 0.50 g
b 1.40 g
c 5.49 g
d 10.90 g
e 1.90 g
give me an explanation on why it is the correct answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 06:30
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1
You know the right answer?
Asample of cesium carbonate, weighing 3.80 g, requires 1.90 g of hydrogen bromide gas to completely...
Questions

Social Studies, 05.01.2021 23:40

Business, 05.01.2021 23:40

Mathematics, 05.01.2021 23:40

Biology, 05.01.2021 23:40



Social Studies, 05.01.2021 23:40

Chemistry, 05.01.2021 23:40

Social Studies, 05.01.2021 23:40

Mathematics, 05.01.2021 23:40

Mathematics, 05.01.2021 23:40

Arts, 05.01.2021 23:40

Mathematics, 05.01.2021 23:40



Mathematics, 05.01.2021 23:40

Mathematics, 05.01.2021 23:40


Health, 05.01.2021 23:40

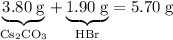 .
. represent the mass of carbon dioxide produced in this reaction.
represent the mass of carbon dioxide produced in this reaction.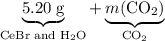 .
.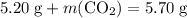 .
. .
.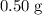 .
.


