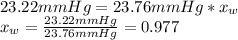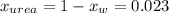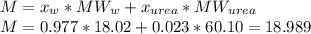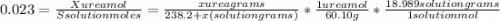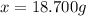Chemistry, 16.07.2019 04:10 reginaldlegette
The vapor pressure of water is 23.76 mm hg at 25°c. how many grams of urea, ch4n2o, a nonvolatile, nonelectrolyte (mw = 60.10 g/mol), must be added to 238.2 grams of water to reduce the vapor pressure to 23.22 mm hg ? water = h2o = 18.02 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
The vapor pressure of water is 23.76 mm hg at 25°c. how many grams of urea, ch4n2o, a nonvolatile, n...
Questions

Mathematics, 17.12.2020 09:10

Mathematics, 17.12.2020 09:10


English, 17.12.2020 09:10

Mathematics, 17.12.2020 09:10

History, 17.12.2020 09:10







Mathematics, 17.12.2020 09:10

Chemistry, 17.12.2020 09:10




Health, 17.12.2020 09:10

Biology, 17.12.2020 09:10

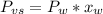
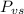 is the vapor pressure of the solution,
is the vapor pressure of the solution, 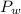 is the vapor pressure of the pure water, and
is the vapor pressure of the pure water, and 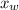 is the molar fraction of water. This equation applies just for that kind of solutes and at low pressures (23.76 mmHg is a low pressure).
is the molar fraction of water. This equation applies just for that kind of solutes and at low pressures (23.76 mmHg is a low pressure).