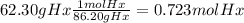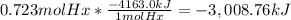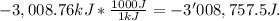Chemistry, 15.07.2019 19:30 wardlawshaliyah
The combustion of hexane is given by the following reaction. 2 c6h14 + 19 o2 12 co2 + 14 h2o the enthalpy of reaction is −4163.0 kj/mol. how much energy (in joules) will be released if 62.30 grams of hexane is burned. (molar mass of hexane = 86.20 g/mol).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2
You know the right answer?
The combustion of hexane is given by the following reaction. 2 c6h14 + 19 o2 12 co2 + 14 h2o the ent...
Questions


History, 26.06.2019 04:00

History, 26.06.2019 04:00



Mathematics, 26.06.2019 04:00

Chemistry, 26.06.2019 04:00




Chemistry, 26.06.2019 04:00

English, 26.06.2019 04:00


Geography, 26.06.2019 04:00

Mathematics, 26.06.2019 04:00


Mathematics, 26.06.2019 04:00

Mathematics, 26.06.2019 04:00

Mathematics, 26.06.2019 04:00