Chemistry, 13.07.2019 23:20 roxymiller3942
The standard cell potential ec for the reduction of silver ions with elemental copper is 0.46v at 25 degrees celsius. calculate δg for this reaction.
*** explain the reactions since i’m very confused as to wich side i should put the electrons.
ex: cu-> cu2+ + 2e

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

You know the right answer?
The standard cell potential ec for the reduction of silver ions with elemental copper is 0.46v at 25...
Questions









Health, 14.09.2019 08:20

Biology, 14.09.2019 08:20


History, 14.09.2019 08:20

Health, 14.09.2019 08:20

History, 14.09.2019 08:20


English, 14.09.2019 08:20




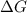 for this reaction is, -88780 J/mole.
for this reaction is, -88780 J/mole.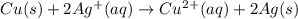
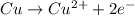
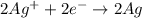
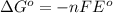
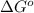 = Gibbs free energy = ?
= Gibbs free energy = ?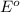 = standard e.m.f of cell = 0.46 V
= standard e.m.f of cell = 0.46 V