
Chemistry, 13.07.2019 22:20 highschoolboy
The fraction of a radioactive isotope remaining at time t is (1/2)^t/t1/2 where t1/2 is the half-life. if the half-life of carbon−14 is 5,730 yr, what fraction of carbon−14 in a piece of charcoal remains after
(a) 14.0 yr?
(b) 1.900 × 10^4 yr? × 10 (enter your answer in scientific notation.)
(c) 1. × 10^5 yr? × 10 (enter your answer in scientific notation.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
The fraction of a radioactive isotope remaining at time t is (1/2)^t/t1/2 where t1/2 is the half-lif...
Questions

Mathematics, 01.04.2020 17:39

Mathematics, 01.04.2020 17:39

Health, 01.04.2020 17:39

Mathematics, 01.04.2020 17:39


Social Studies, 01.04.2020 17:39

Physics, 01.04.2020 17:39



History, 01.04.2020 17:39

Mathematics, 01.04.2020 17:39

Mathematics, 01.04.2020 17:39

History, 01.04.2020 17:39



Biology, 01.04.2020 17:39


Social Studies, 01.04.2020 17:39

Biology, 01.04.2020 17:39

 fraction of carbon−14 in a piece of charcoal remains after 14.0 years.
fraction of carbon−14 in a piece of charcoal remains after 14.0 years.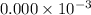 fraction of carbon−14 in a piece of charcoal remains after
fraction of carbon−14 in a piece of charcoal remains after 
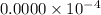 fraction of carbon−14 in a piece of charcoal remains after
fraction of carbon−14 in a piece of charcoal remains after 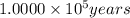 .
.![[A]=\frac{(\frac{1}{2})^t}{t_{\frac{1}{2}}}](/tpl/images/0086/3350/d6525.png)
![\log [A]=t\log[\frac{1}{2}]-\log [t_{\frac{1}{2}}]](/tpl/images/0086/3350/f81b0.png)
 = half life of the carbon−14 =5,730 years
= half life of the carbon−14 =5,730 years![\log [A]= 14 years\times (-3010)-\log [5,730 years]](/tpl/images/0086/3350/51128.png)
![[A]=1.065\times 10^{-8}](/tpl/images/0086/3350/2e677.png)
![\log [A]= 1.900\times 10^4 years\times (-3010)-\log [5,730 years]](/tpl/images/0086/3350/5f99c.png)
![[A]=0.000\times 10^{-3} [/tex](/tpl/images/0086/3350/ae3f2.png)
![\log [A]= 1.0000\times 10^5 years\times (-3010)-\log [5,730 years]](/tpl/images/0086/3350/a2485.png)
![[A]=0.0000\times 10^{-4}](/tpl/images/0086/3350/e1222.png)


