
Chemistry, 13.07.2019 07:10 gabriellabadon2
How many moles of co2 are produced when 45.0g of c6h6 react completely in the following equation? 2c6h6+ 15o2 → 12co2 + 6h2o

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

You know the right answer?
How many moles of co2 are produced when 45.0g of c6h6 react completely in the following equation? 2...
Questions


Chemistry, 01.04.2021 23:30


English, 01.04.2021 23:30

Mathematics, 01.04.2021 23:30

Computers and Technology, 01.04.2021 23:30




Mathematics, 01.04.2021 23:30

English, 01.04.2021 23:30

Biology, 01.04.2021 23:30

Mathematics, 01.04.2021 23:30




Mathematics, 01.04.2021 23:40



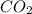 produced are, 3.462 moles.
produced are, 3.462 moles.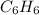 = 45.0 g
= 45.0 g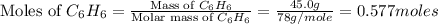
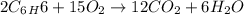
 moles of
moles of 

