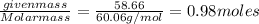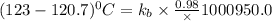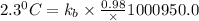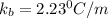Chemistry, 12.07.2019 20:30 joannegrace869
The normal boiling point of a certain liquid x is 120.7°c , but when 58.66g of urea nh22co are dissolved in 950.g of x , it is found that the solution boils at 123.0°c instead. use this information to calculate the molal boiling point elevation constant kb of x .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1


Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

You know the right answer?
The normal boiling point of a certain liquid x is 120.7°c , but when 58.66g of urea nh22co are disso...
Questions

Computers and Technology, 26.11.2019 21:31









History, 26.11.2019 21:31





Biology, 26.11.2019 21:31

Mathematics, 26.11.2019 21:31




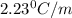
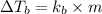
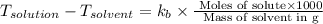
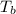 = change in boiling point
= change in boiling point
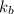 = boiling point constant
= boiling point constant