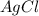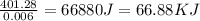Chemistry, 12.07.2019 20:20 jjjones2233
30.0 ml of 0.20 m agno, are added to 100.0 ml of 0.10 m hci in a thermally nsulated vessel. the following reaction takes place: ag (aq)+ cl (aq)agci (s) the two solutions were initially at 22.00°c and the final temperature was 22.80 c calculate the heat of this reaction in k. jimol of agci formed. assume a combined mass of 120 g and a specific heat capacity of 4.18 jk-'g for the reaction mixture.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1


Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1
You know the right answer?
30.0 ml of 0.20 m agno, are added to 100.0 ml of 0.10 m hci in a thermally nsulated vessel. the foll...
Questions










Mathematics, 30.03.2020 20:30

Health, 30.03.2020 20:30






Mathematics, 30.03.2020 20:30

History, 30.03.2020 20:30

Mathematics, 30.03.2020 20:30

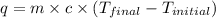
 = specific heat capacity =
= specific heat capacity = 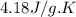
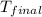 = final temperature =
= final temperature = 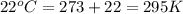
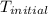 = initial temperature =
= initial temperature = 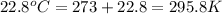
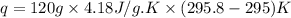
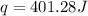
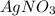 and
and 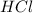 .
.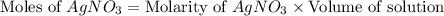
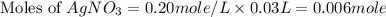
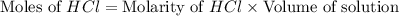
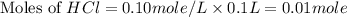
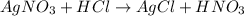
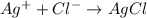
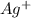 react with 1 mole
react with 1 mole 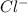 to produce 1 mole of
to produce 1 mole of