

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
The combustion of propane (c3h8) produces co2 and h2o: c3h8 (g) 5o2 (g) → 3co2 (g) 4h2o (g) the rea...
Questions


Chemistry, 11.05.2021 09:50


Mathematics, 11.05.2021 14:00

Physics, 11.05.2021 14:00


Mathematics, 11.05.2021 14:00


Mathematics, 11.05.2021 14:00

Mathematics, 11.05.2021 14:00

Mathematics, 11.05.2021 14:00

Business, 11.05.2021 14:00


Chemistry, 11.05.2021 14:00

English, 11.05.2021 14:00

Computers and Technology, 11.05.2021 14:00

History, 11.05.2021 14:00



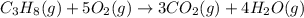
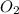 produces = 4 moles of
produces = 4 moles of 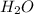
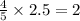 moles of
moles of 

