
Chemistry, 12.07.2019 02:20 Cupcake8189
Part 1. determine the molar mass of a 0.622-gram sample of gas having a volume of 2.4 l at 287 k and 0.850 atm. show your work.
part 2. if this sample was placed under extremely low temperature, describe how the actual volume would compare to the predicted volume. explain your answer.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1


Chemistry, 23.06.2019 11:00
Nh4no3 n2o + 2h2o a chemist who is performing this reaction starts with 160.1 g of nh4no3. the molar mass of nh4no3 is 80.03 g/mol; the molar mass of water (h2o) is 18.01 g/mol. what mass, in grams, of h2o is produced?
Answers: 1
You know the right answer?
Part 1. determine the molar mass of a 0.622-gram sample of gas having a volume of 2.4 l at 287 k and...
Questions


History, 04.02.2020 21:46

History, 04.02.2020 21:46





Mathematics, 04.02.2020 21:46


Biology, 04.02.2020 21:46


History, 04.02.2020 21:46

Chemistry, 04.02.2020 21:46




History, 04.02.2020 21:46

History, 04.02.2020 21:46

Mathematics, 04.02.2020 21:46

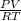
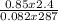
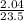 = 0.087mole
= 0.087mole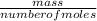
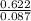 =7.15gmol⁻¹
=7.15gmol⁻¹

