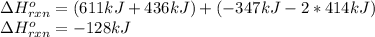Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Read the given expression. x = number of protons − number of core electrons which of the following explains the identity of x and its trends across a period? x is the effective nuclear charge, and it remains constant across a period. x is the screening constant, and it remains constant across a period. x is the effective nuclear charge, and it increases across a period. x is the screening constant, and it increases across a period.
Answers: 1

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

You know the right answer?
Use the bond energies provided to estimate δh°rxn for the reaction below. c2h4(g) + h2(g) → c2h6(g)...
Questions




History, 17.03.2020 23:36

Mathematics, 17.03.2020 23:36



Mathematics, 17.03.2020 23:36

Biology, 17.03.2020 23:36


Physics, 17.03.2020 23:36




Social Studies, 17.03.2020 23:36



History, 17.03.2020 23:36


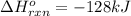
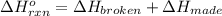
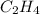 a double bond between carbons is broken as well as a bond between hydrogens (such values turn out positive). Furthermore, a single bond between carbons and two single bonds between carbon and hydrogen are made (such values turn out negative), in such a way, we develop the aforesaid equation to obtain:
a double bond between carbons is broken as well as a bond between hydrogens (such values turn out positive). Furthermore, a single bond between carbons and two single bonds between carbon and hydrogen are made (such values turn out negative), in such a way, we develop the aforesaid equation to obtain: