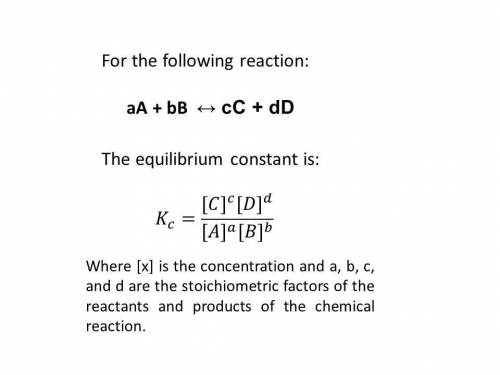
Consider the following reaction and its equilibrium constant: 4 cuo(s) + ch4(g) ⇌ co2(g) + 4 cu(s) + 2 h2o(g) kc = 1.10.
a reaction mixture contains 0.22 m ch4, 0.70 m co2 and 1.5 m h2o. which of the following statements is true concerning this system? a. the reaction will shift in the direction of products. b. the equilibrium constant will increase. c. the reaction will shift in the direction of reactants. d. the system is at equilibrium.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Which of these will change if the air in aclosed bottle is heated? abcdthe mass of the airthe composition of the airthe air pressure in the bottlethe number of air molecules in the bottle
Answers: 3

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
Consider the following reaction and its equilibrium constant: 4 cuo(s) + ch4(g) ⇌ co2(g) + 4 cu(s)...
Questions


Mathematics, 17.01.2022 23:30

Mathematics, 17.01.2022 23:30

English, 17.01.2022 23:30


English, 17.01.2022 23:30





Mathematics, 17.01.2022 23:30

Mathematics, 17.01.2022 23:30

Mathematics, 17.01.2022 23:30




History, 17.01.2022 23:30

English, 17.01.2022 23:30

Mathematics, 17.01.2022 23:40

Mathematics, 17.01.2022 23:40

![Q = \frac{[CO_{2}][H_{2}O]^{2}}{[CH_{4}]}](/tpl/images/0072/0904/ac4b6.png) (2)
(2)![Q = \frac{[CO_{2}][H_{2}O]^{2}}{[CH_{4}]} = \frac{0.70*(1.5)^{2}}{0.22} = 7.16](/tpl/images/0072/0904/5a31b.png)