

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3


Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
Which of the following is an oxidation-reduction reaction? fe2o3 + 3co mc009-1.jpg 2fe + 3co2 cuso4...
Questions




Chemistry, 18.05.2021 22:50


Social Studies, 18.05.2021 22:50

Social Studies, 18.05.2021 22:50



Mathematics, 18.05.2021 22:50








Health, 18.05.2021 22:50


Mathematics, 18.05.2021 22:50

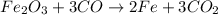 is an oxidation-reduction reaction.
is an oxidation-reduction reaction.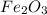 +3
+3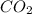 +4
+4

