
Ammonia is a principal nitrogen fertilizer. it is prepared by the reaction between hydrogen and nitrogen. 3h2(g) + n2(g) → 2nh3(g) in a particular reaction, 8.00 moles of nh3 were produced. how many moles of h2 and how many moles of n2 were reacted to produce this amount of nh3?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
Ammonia is a principal nitrogen fertilizer. it is prepared by the reaction between hydrogen and nitr...
Questions

English, 14.02.2020 05:28



Mathematics, 14.02.2020 05:28







Mathematics, 14.02.2020 05:28



Spanish, 14.02.2020 05:28






Mathematics, 14.02.2020 05:28

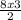 = 12moles
= 12moles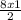 = 4moles of Nitrogen gas
= 4moles of Nitrogen gas

