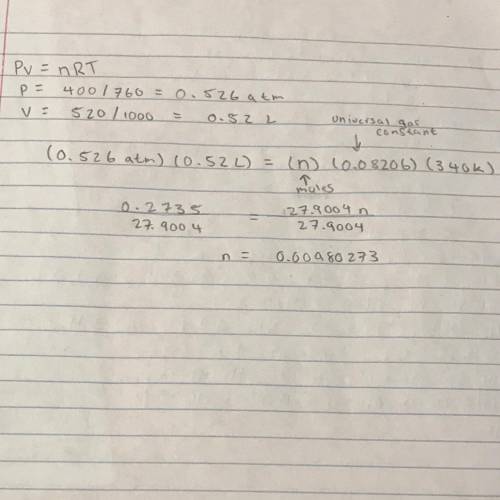Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Noble gases are the most reactive elements on the periodic table. a. true b. false
Answers: 2

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1
You know the right answer?
How many moles of gas does it take to occupy 520 ml at a pressure of 400 torr and a temperature of 3...
Questions

Computers and Technology, 14.10.2019 11:00

History, 14.10.2019 11:00



Mathematics, 14.10.2019 11:00

History, 14.10.2019 11:00


English, 14.10.2019 11:00

Mathematics, 14.10.2019 11:00

Mathematics, 14.10.2019 11:00




Biology, 14.10.2019 11:00

History, 14.10.2019 11:00

Mathematics, 14.10.2019 11:00

Mathematics, 14.10.2019 11:00

Mathematics, 14.10.2019 11:00


Chemistry, 14.10.2019 11:00