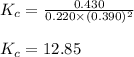Chemistry, 09.07.2019 04:30 dakotalynnwillis01
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.350 m , [b] = 0.650 m , and [c] = 0.300 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.220 m and [c] = 0.430 m . calculate the value of the equilibrium constant, kc.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.350 m , [b] = 0.65...
Questions


History, 07.04.2021 06:20

Mathematics, 07.04.2021 06:20


Health, 07.04.2021 06:20

Mathematics, 07.04.2021 06:20



Mathematics, 07.04.2021 06:20


Biology, 07.04.2021 06:20

English, 07.04.2021 06:20

Mathematics, 07.04.2021 06:20


English, 07.04.2021 06:30


Mathematics, 07.04.2021 06:30



Mathematics, 07.04.2021 06:30

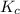 for the given reaction is 12.85.
for the given reaction is 12.85.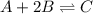
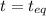 (0.350 - x) (0.650 - 2x) (0.300 + x)
(0.350 - x) (0.650 - 2x) (0.300 + x)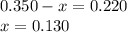
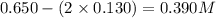
![K_c=\frac{[C]}{[A][B]^2}](/tpl/images/0068/1022/240ef.png)