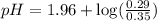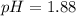Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Question 5 a solution is prepared at 25°c that is initially 0.35m in chlorous acid hclo2, a weak aci...
Questions

History, 28.12.2019 17:31

English, 28.12.2019 17:31


Mathematics, 28.12.2019 17:31


Mathematics, 28.12.2019 17:31


Mathematics, 28.12.2019 17:31


Mathematics, 28.12.2019 17:31

Biology, 28.12.2019 17:31


Mathematics, 28.12.2019 17:31

Mathematics, 28.12.2019 17:31

Arts, 28.12.2019 17:31

History, 28.12.2019 17:31

Business, 28.12.2019 17:31

English, 28.12.2019 17:31


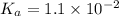
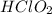 = 0.35 M
= 0.35 M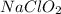 = 0.29 M
= 0.29 M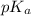 .
.![pK_a=-\log [K_a]](/tpl/images/0067/9400/2d95a.png)
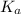 in this expression, we get:
in this expression, we get: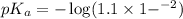
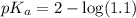
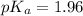
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0067/9400/e961a.png)
![pH=pK_a+\log \frac{[NaClO_2]}{[HClO_2]}](/tpl/images/0067/9400/a8df0.png)