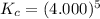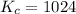Chemistry, 09.07.2019 03:20 chrismcb875
Determine the value of kc for the following reaction if the equilibrium concentrations are as follows: [p4o10]eq = 2.000 moles, [p4]eq = 3.000 moles, [o2]eq = 4.000 m p4o10(s) ↔ p4(s) + 5 o2(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2
You know the right answer?
Determine the value of kc for the following reaction if the equilibrium concentrations are as follow...
Questions


Biology, 14.02.2020 07:46

Biology, 14.02.2020 07:46


Mathematics, 14.02.2020 07:46

Mathematics, 14.02.2020 07:46


Mathematics, 14.02.2020 07:46




Mathematics, 14.02.2020 07:47

Mathematics, 14.02.2020 07:47

Physics, 14.02.2020 07:47

Mathematics, 14.02.2020 07:47

Physics, 14.02.2020 07:48

English, 14.02.2020 07:49

Mathematics, 14.02.2020 07:49


English, 14.02.2020 07:49

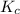 for the following reaction will be, 1024
for the following reaction will be, 1024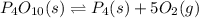
![K_c=[O_2]^5](/tpl/images/0067/9324/32ee9.png)