
Chemistry, 09.07.2019 02:10 Nathaliasmiles
The concentration of rn−222 in the basement of a house is 1.45 × 10−6 mol/l. assume the air remains static and calculate the concentration of the radon after 3.00 days. the half-life of rn−222 is 3.82 days.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1
You know the right answer?
The concentration of rn−222 in the basement of a house is 1.45 × 10−6 mol/l. assume the air remains...
Questions


Mathematics, 20.04.2021 02:20

Mathematics, 20.04.2021 02:20


Mathematics, 20.04.2021 02:20



English, 20.04.2021 02:20

Arts, 20.04.2021 02:20

Mathematics, 20.04.2021 02:20

Social Studies, 20.04.2021 02:20

English, 20.04.2021 02:20

Mathematics, 20.04.2021 02:20



Mathematics, 20.04.2021 02:20


Mathematics, 20.04.2021 02:20

Mathematics, 20.04.2021 02:20

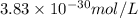
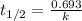
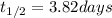
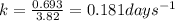
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0067/7648/f1041.png)
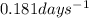
![[A_o]](/tpl/images/0067/7648/dc622.png) = initial amount of the reactant =
= initial amount of the reactant = 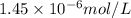
![0.181days^{-1}=\frac{2.303}{3.00days}\log\frac{1.45\times 10^{-6}}{[A]}](/tpl/images/0067/7648/19acc.png)
![[A]=3.83\times 10^{-30}mol/L](/tpl/images/0067/7648/00760.png)



