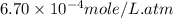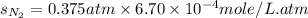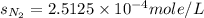Chemistry, 09.07.2019 01:30 glydelxc2780
Calculate the solubility of nitrogen in water at an atmospheric pressure of 0.480 atm (a typical value at high altitude). atmospheric gas mole fraction kh mol/(l*atm) n2 7.81 x 10-1 6.70 x 10-4 o2 2.10 x 10-1 1.30 x 10-3 ar 9.34 x 10-3 1.40 x 10-3 co2 3.33 x 10-4 3.50 x 10-2 ch4 2.00 x 10-6 1.40 x 10-3 h2 5.00 x 10-7 7.80 x 10-4

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
You know the right answer?
Calculate the solubility of nitrogen in water at an atmospheric pressure of 0.480 atm (a typical val...
Questions



Mathematics, 07.09.2019 04:20

Mathematics, 07.09.2019 04:20


History, 07.09.2019 04:20

English, 07.09.2019 04:20

History, 07.09.2019 04:20












Computers and Technology, 07.09.2019 04:20

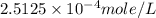
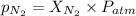
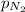 = partial pressure of nitrogen = ?
= partial pressure of nitrogen = ?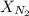 = mole fraction of nitrogen =
= mole fraction of nitrogen = 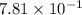
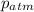 = atmospheric pressure = 0.480 atm
= atmospheric pressure = 0.480 atm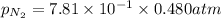
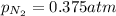
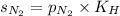
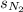 = solubility of nitrogen in water = ?
= solubility of nitrogen in water = ?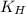 = Henry's constant =
= Henry's constant =