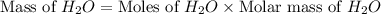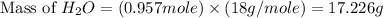Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Does anyone know a lot about how to: - calculate mass of magnesium metal - calculate the actual yield of magnesium oxide - calculate the theoretical yield of mgo - calculate the percent yield of mgo - determine the percent yield of mgo - determine the average percent yield of mgo i had to do an online lab and its asking these questions but i have no idea where to start or how to be able to find these things. i can post the chart of the data from the lab or if you can tell me exactly how i can find each.
Answers: 3

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1


You know the right answer?
What is the percent yield of a reaction in which 74.1 g of tungsten(vi) oxide (wo3) reacts with exce...
Questions




English, 25.06.2021 16:30

Social Studies, 25.06.2021 16:30



Mathematics, 25.06.2021 16:30





English, 25.06.2021 16:40




Computers and Technology, 25.06.2021 16:40



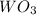 = 74.1 g
= 74.1 g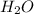 = 18 g/mole
= 18 g/mole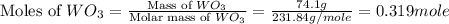
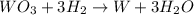
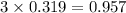 moles of
moles of