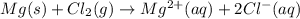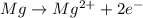Chemistry, 08.07.2019 18:30 mattsucre1823
Identify the oxidized substance, the reduced substance, the oxidizing agent, and the reducing agent in the redox reaction. mg(s)+cl2(g)⟶mg2+(aq)+2cl−(aq) which substance gets oxidized? mg2+ cl− mg cl2 which substance gets reduced? cl2 mg cl− mg2+ what is the oxidizing agent? cl− mg2+ cl2 mg what is the reducing agent? mg2+ cl2 mg cl−

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
Identify the oxidized substance, the reduced substance, the oxidizing agent, and the reducing agent...
Questions




Mathematics, 17.08.2020 06:01

Social Studies, 17.08.2020 06:01




Mathematics, 17.08.2020 06:01





Mathematics, 17.08.2020 06:01






History, 17.08.2020 06:01