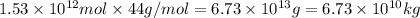Calcium oxide or quicklime (cao) is used in steelmaking, cement manufacture, and pollution control. it is prepared by the thermal decomposition of calcium carbonate: caco3(s) → cao(s) co2(g) calculate the yearly release of co2 (in kg) to the atmosphere if the annual production of cao in the united states is 8.6 × 1010 kg.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1


Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
Calcium oxide or quicklime (cao) is used in steelmaking, cement manufacture, and pollution control....
Questions



Mathematics, 05.05.2020 13:53


Spanish, 05.05.2020 13:53

Mathematics, 05.05.2020 13:53



Mathematics, 05.05.2020 13:53

English, 05.05.2020 13:53



Mathematics, 05.05.2020 13:53

Mathematics, 05.05.2020 13:53


Physics, 05.05.2020 13:53

Mathematics, 05.05.2020 13:54



Mathematics, 05.05.2020 13:54

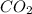 into the atmosphere is
into the atmosphere is 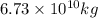 .
.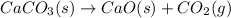
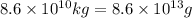
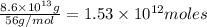
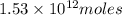 of CaO moles of carbon-dioxide moles produced will be:
of CaO moles of carbon-dioxide moles produced will be: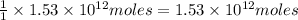 of carbon-dioxide
of carbon-dioxide moles of carbon-dioxide:
moles of carbon-dioxide: