
Chemistry, 05.02.2020 12:00 hillisaiah734
The reform reaction between steam and gaseous methane (ch4) produces "synthesis gas," a mixture of carbon monoxide gas and dihydrogen gas. synthesis gas is one of the most widely used industrial chemicals, and is the major industrial source of hydrogen.
suppose a chemical engineer studying a new catalyst for the reform reaction finds that 924. liters per second of methane are consumed when the reaction is run at 261.°c and 0.96atm. calculate the rate at which dihydrogen is being produced. give your answer in kilograms per second. round your answer to 2 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
The reform reaction between steam and gaseous methane (ch4) produces "synthesis gas," a mixture of c...
Questions



History, 23.07.2019 19:00




English, 23.07.2019 19:00

Mathematics, 23.07.2019 19:00

Biology, 23.07.2019 19:00

English, 23.07.2019 19:00

Biology, 23.07.2019 19:00

Mathematics, 23.07.2019 19:00




Social Studies, 23.07.2019 19:00



Social Studies, 23.07.2019 19:00

History, 23.07.2019 19:00

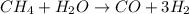 Haber reaction
Haber reaction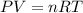
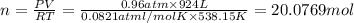
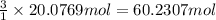 of dihydrogen
of dihydrogen

