
Chemistry, 08.07.2019 17:30 meramera50
Be sure to answer all parts. propane (c3h8) is a minor component of natural gas and is used in domestic cooking and heating. (a) balance the following equation representing the combustion of propane in air. include states of matter in your answer. c3h8(g) + o2(g) → co2(g) + h2o(g) (b) how many grams of carbon dioxide can be produced by burning 8.11 moles of propane? assume that oxygen is the excess reactant in this reaction. × 10 g enter your answer in scientific notation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3


Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2
You know the right answer?
Be sure to answer all parts. propane (c3h8) is a minor component of natural gas and is used in domes...
Questions

Mathematics, 16.09.2020 21:01

Mathematics, 16.09.2020 21:01

Mathematics, 16.09.2020 21:01

Mathematics, 16.09.2020 21:01

Mathematics, 16.09.2020 21:01

English, 16.09.2020 21:01

Mathematics, 16.09.2020 21:01

Mathematics, 16.09.2020 21:01

Mathematics, 16.09.2020 21:01

Mathematics, 16.09.2020 21:01

Mathematics, 16.09.2020 21:01

Mathematics, 16.09.2020 21:01

English, 16.09.2020 21:01

Mathematics, 16.09.2020 21:01

Physics, 16.09.2020 21:01

Mathematics, 16.09.2020 21:01

Mathematics, 16.09.2020 21:01

Geography, 16.09.2020 21:01

English, 16.09.2020 21:01

Mathematics, 16.09.2020 21:01

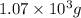
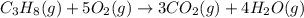
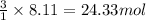 of carbon dioxide gas.
of carbon dioxide gas.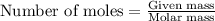
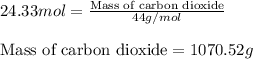
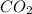 produced in the given reaction and expressed in scientific notation is
produced in the given reaction and expressed in scientific notation is 

