
Chemistry, 10.10.2019 12:00 savannahvargas512
Compounds a and b react to form compounds c and d according to the equation: aa + bb → cc + dd. under which conditions will the rate law be given by the equation: rate = k[a]a[b]b? a. the reaction takes place in one step. b. the reaction is endothermic. c. the reaction is exothermic. d. the reaction involves more than one step.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3

You know the right answer?
Compounds a and b react to form compounds c and d according to the equation: aa + bb → cc + dd. und...
Questions



English, 30.06.2020 05:01

History, 30.06.2020 05:01

Mathematics, 30.06.2020 05:01






Biology, 30.06.2020 05:01

Physics, 30.06.2020 05:01

Social Studies, 30.06.2020 05:01

Mathematics, 30.06.2020 05:01





Mathematics, 30.06.2020 05:01

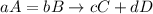
![Rate=k[A]^a[B]^b](/tpl/images/0306/8163/8c0b1.png)


